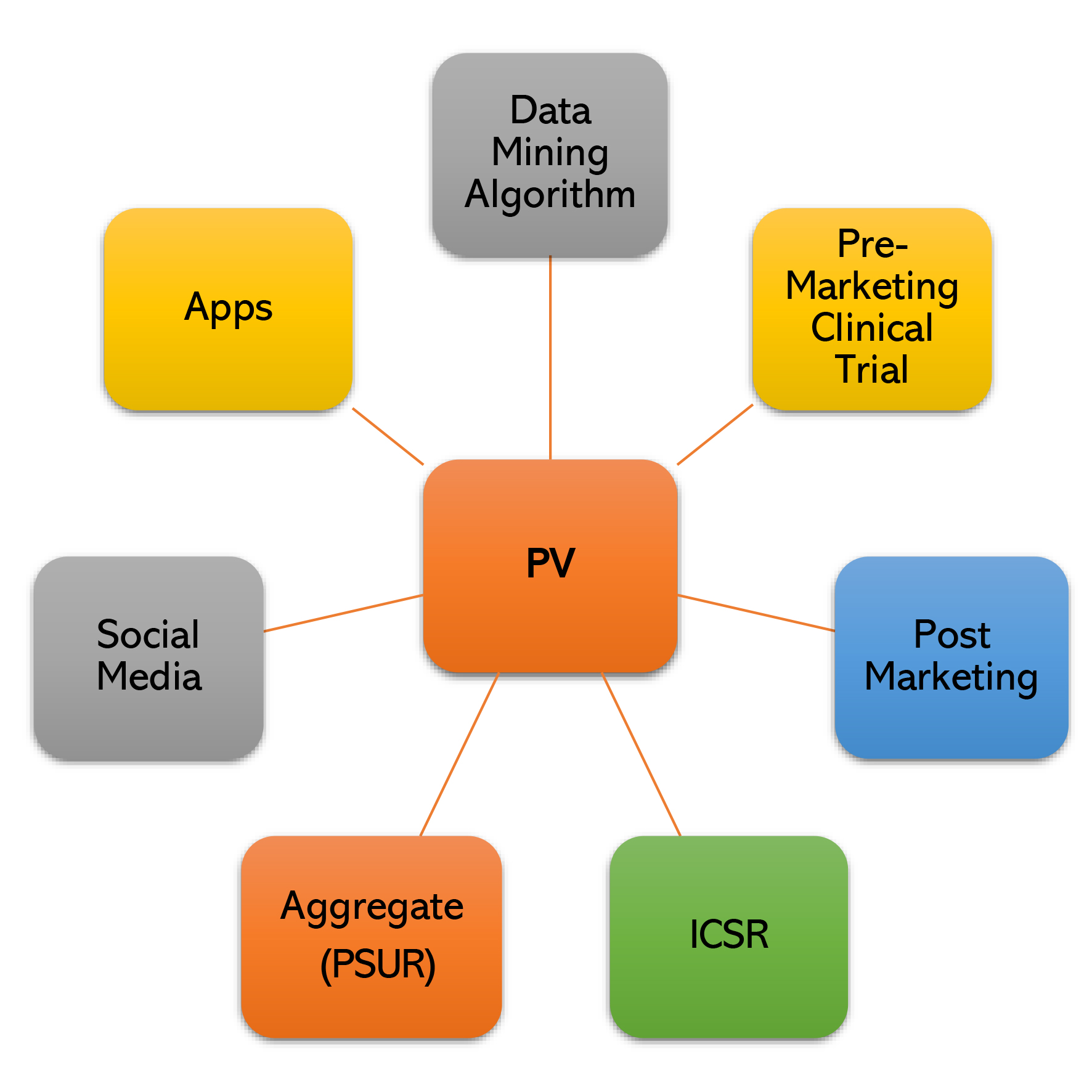
End-to-End ‘Drug Safety Data Management’ through ‘Latest AI based Drug Safety Database’ with ‘Fully Automated Literature & Signal Detection’ activities compliant to ’21 CFR (US) & EMA’.
Under the guidance of most eminent personality, “Dr. Rajinder K. Jalali”, who has served for more than 18 years as “Vice President & Head, Medical Affairs & Clinical Research” and “Global Head Pharmacovigilance” in Ranbaxy and Sun Pharma. Major services covered are: ICSR Case Processing, · E2b (R2), E2b (R3) Submissions,· Drug Safety Writing – PBRER, PSUR PADER,Literature Surveillance (Fully Automated), Signal Detection (Fully Automated), RMP Management, SDEA Management, PSMF Development, Safety Data Migration , Quality Backlog clearing (end-to-end) , AE/ADR/Product Quality Complaint Management,SOP & Working Instructions, Global Compliance monitoring, Audits & Inspections Management
Only logged in customers who have purchased this product may leave a review.
Indivirtus Group of Companies
Need Help? Contact Us Leave Feedback
Categories: B2B Research, B2B Services
Tags: PADER, PBRER, Phamacovigilance, PSUR, PV







Reviews
There are no reviews yet.